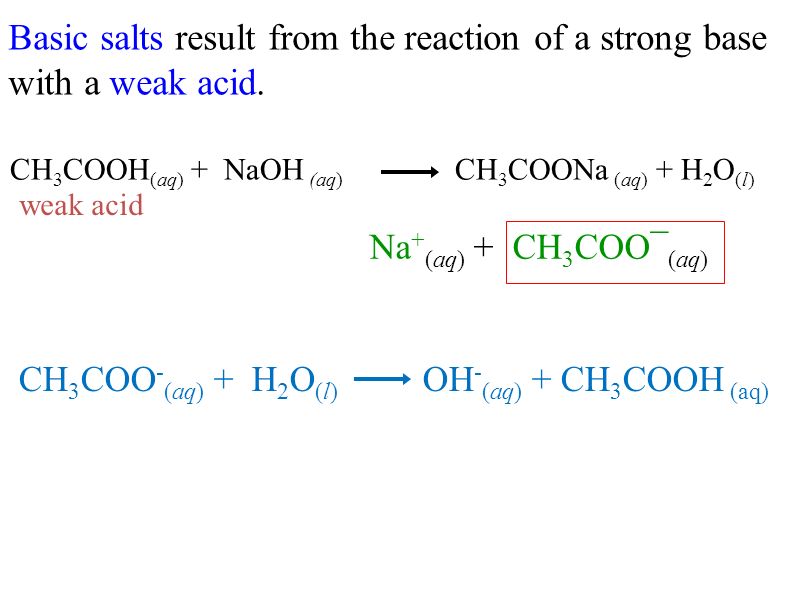
![SOLVED: Theme: Acid-base balance Determine the pH of a solution of sodium acetate [CH3COONa] which is 0.150 M. Then, determine the % hydrolysis SOLVED: Theme: Acid-base balance Determine the pH of a solution of sodium acetate [CH3COONa] which is 0.150 M. Then, determine the % hydrolysis](https://cdn.numerade.com/ask_previews/567a4379-b31b-4987-9817-e40d2cf36c2a_large.jpg)
SOLVED: Theme: Acid-base balance Determine the pH of a solution of sodium acetate [CH3COONa] which is 0.150 M. Then, determine the % hydrolysis

Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.

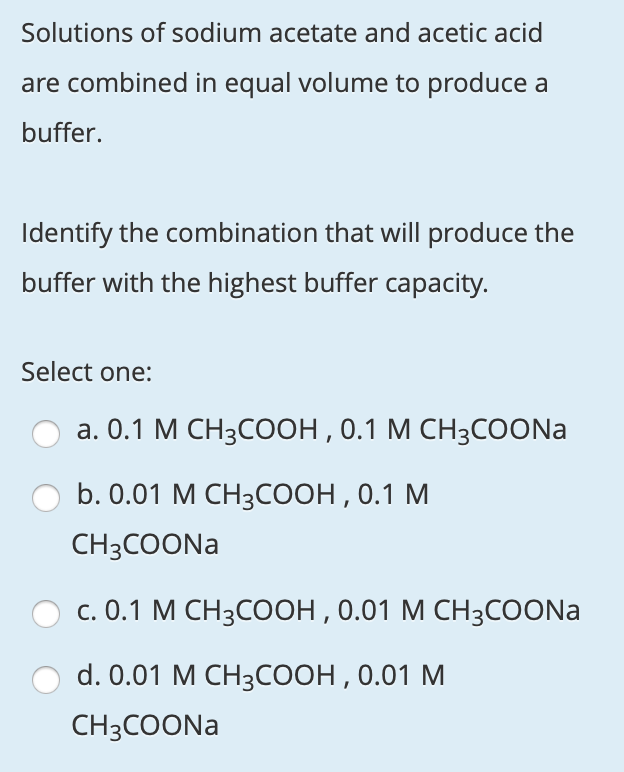
SOLVED: A buffer is prepared using acetic acid, CH3COOH, (a weak acid, pKa = 4.75) and sodium acetate, CH3COONa (which provides acetate ions, the conjugate base), according to the following proportions: Volume
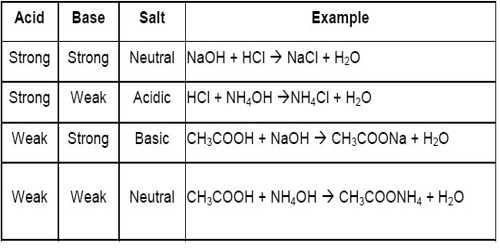
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora