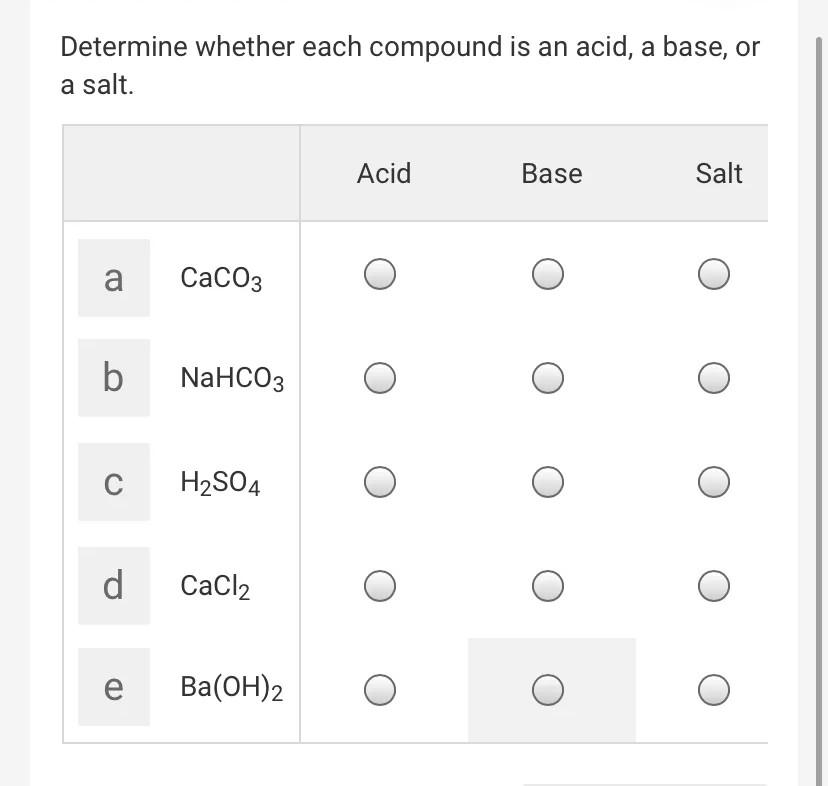
Acid-base reaction of naproxen and calcium carbonate, containing the... | Download Scientific Diagram

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa
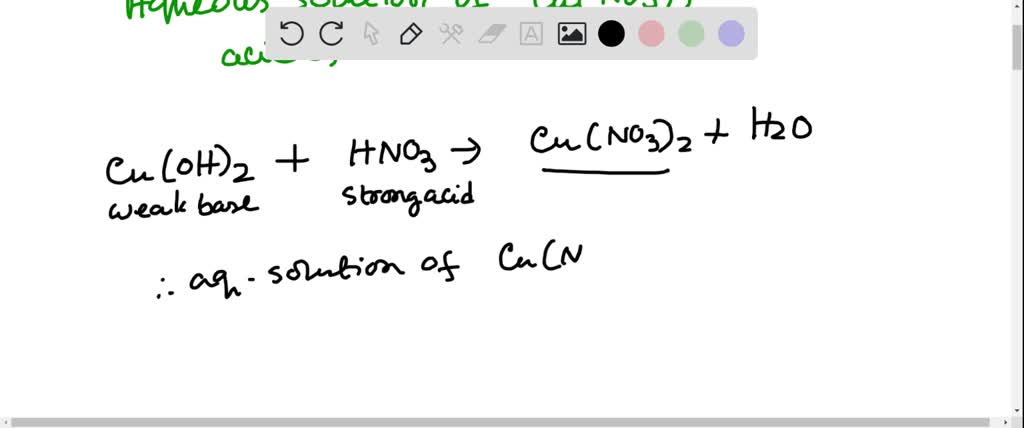
SOLVED: Solid nickel (III) nitrate, Ni(NO3)3, is dissolved in water. Is this solution acidic, basic, or neutral? Solid calcium carbonate, CaCO3, is dissolved in water. Is this solution acidic, basic, or neutral?
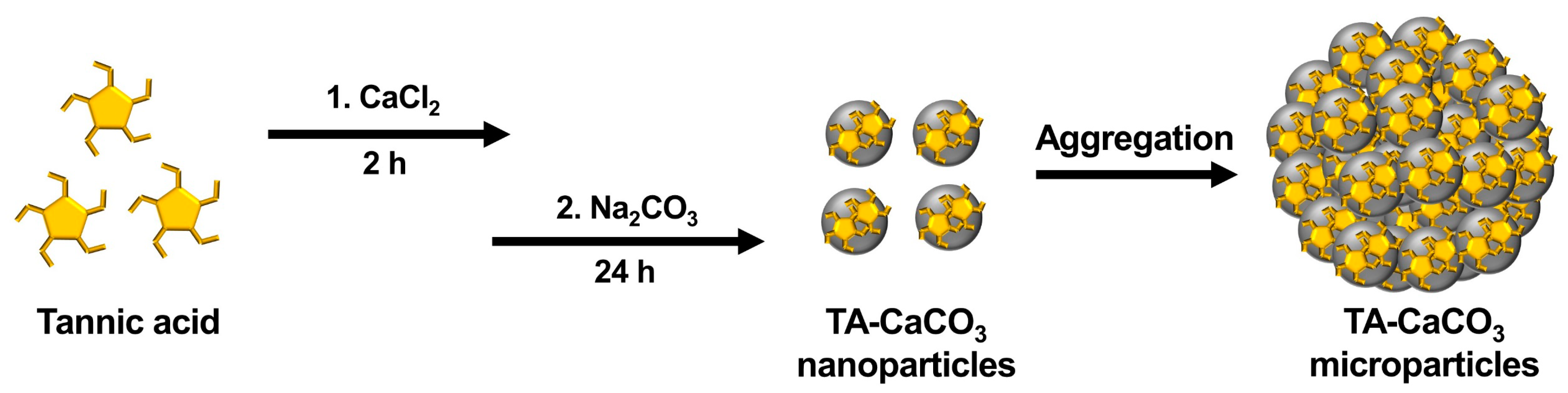
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects

Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + CO2 (g) + H2O (l) .What mass of CaCO3

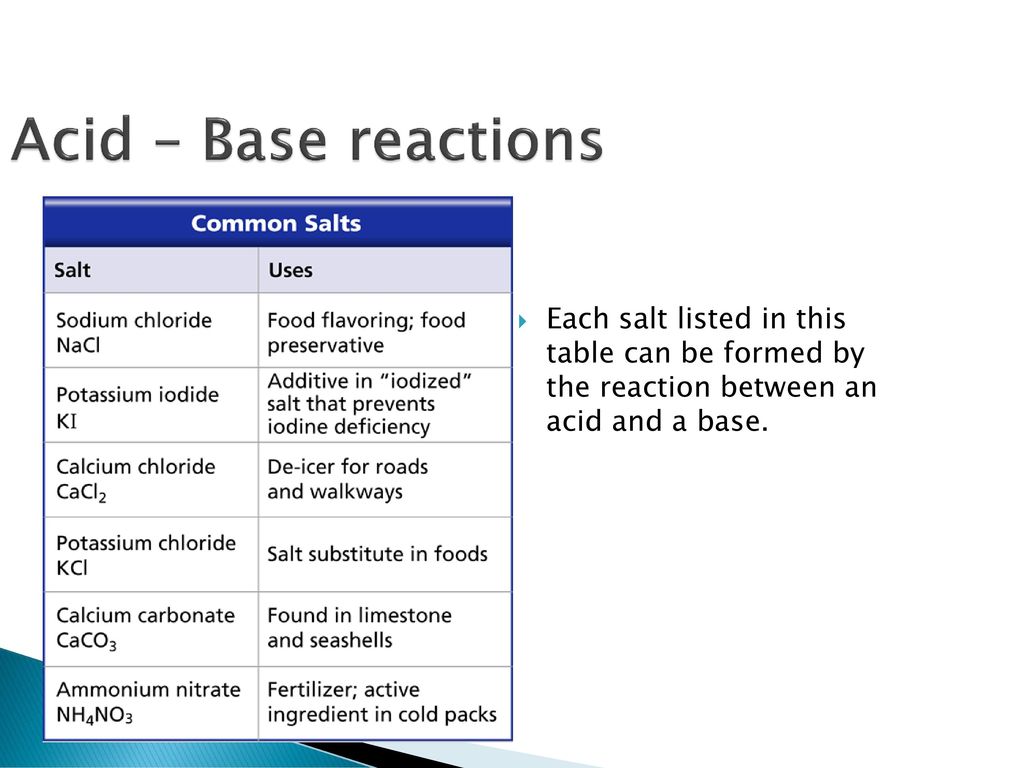
Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download

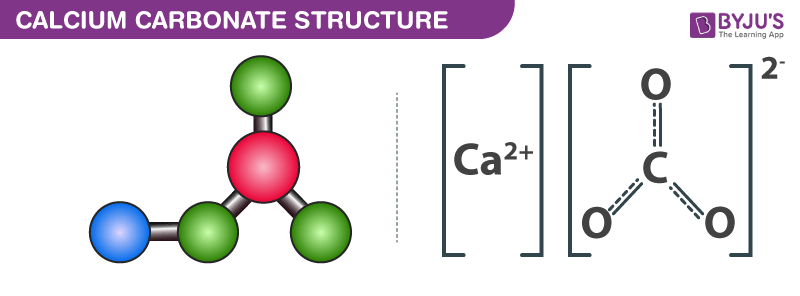
![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)






![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/475685cc-4d58-4e74-a739-8cbc5d1c10f8/q8---parent-acid-and-base-of-calcium-carbonate---teachoo.jpg)